Acetic acid produced by fermentation and oxidation of natural carbohydrates is called vinegar. One of the breakdown products of aspirin is acetic acid (vinegar). Even the slightest decomposition of aspirin will give this smell, even though the aspirin may still be 99+% pure.
The primary reason behind the vinegar-like smell of old aspirin is the presence of acetic acid and salicylic acid, which are yielded when an aspirin undergoes hydrolysis. Moist conditions contribute to its decomposition, especially when people stock it for a very long period, its acid dissociation constant stands at 3.5 at 25 degrees Celsius. Aspirin comprises of acetic acid and salicylic acid. Acetic acid makes up most of the aspirin tablets and therefore smells like vinegar. As aspirin when kept for a long duration of time absorbs moisture in the air is which makes it undergo a chemical transformation to its original components. Many types of aspirin are coated with a substance that prevents this breakdown, but aspirin exposed to the air can begin to smell like vinegar 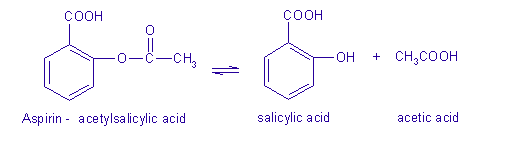
To slow down the decomposition process of aspirin, it must be stored in suitable conditions.
- Store the container in a cool and dry place.
Exposure to moisture will facilitate the hydrolysis of the aspirin molecules into its decomposition products.
- Keep the container tightly closed.
