Explanation:
.......Ethyl chloride can undergo both nucleophilic substitution as well as elimination reactions with strong alkali like KOH.
.......When it undergoes nucleophilic substitution, ethyl alcohol is formed as major product.

.
Mechanism of nucleophilic substitution
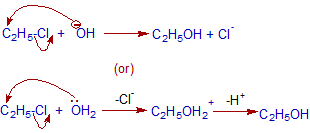
.
Above mechanism depicts SN2 path. Usually primary alkyl halides show Nucleophilic bimolecular substitutions since the formation of carbocation is less easy. Hence bond breaking and making occurs in one step.
However, due to presence of β-hydrogen, ethyl chloride can also undergo elimination reaction to give ethylene in presence of strong base like KOH.

